0 page found
The Imaging and Optics Facility is facilitating the use and maintenance of an wide variety of advanced Microscopy, Imaging analysis and Cytometry devices, as well as software licenses and system accessories. Before anyone can make use of any system, users need to complete a training by the Imaging and Optics Facility Facility team before bookings can be made.
IOF machine-park exists of different imaging technologies and applications, which also come with different levels of complexity. To clarify the complexity of the different systems, we indicate the level of complexity – and the default required training effort – in the following manner:
• Level 0: only requires ‘introduction to Imaging & Optics Facility’ (Stereo; Analysis Workstations, etc)
• Level 1: requires 2 × training → standard Widefield and Confocal applications
• Level 2: requires 2 × training + 1 assisted session / additional training required → Spinning Disk, Multi Modal, Advanced Applications & Assays (FLIM, UV ablation, pipette aspiration, …)
• Level 3: requires 2 × trainings + Assisted Sessions + System configuration by IOF staff* → AFM / Light-sheet* / Multi-photon*
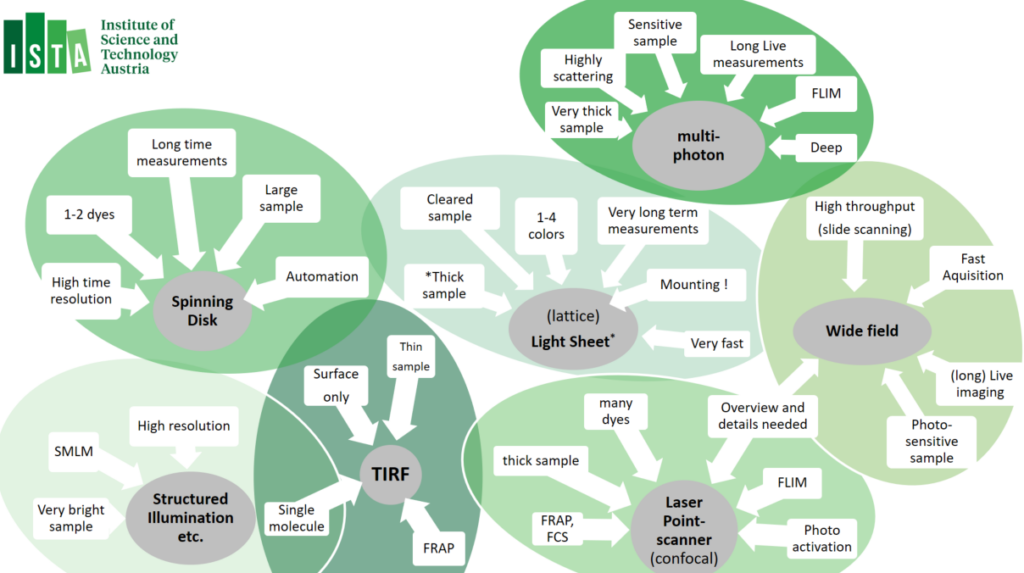
Guidelines:
Grouped Equipment Overview:
Other SSU Instrumentation:
CYTOMETRY
00. Flow Cytometry: Fluorescence-Activated Cell Sorting (FACS)
The Imaging and Optics Facility provides an high-end Flow Cytometry machine park and analysis infrastructure.
MICROSCOPY
01. Widefield Microscopes
Our widefield equipment park consists of automated widefield microscopes, as well as slidescanners. The slidescanner are best used for large volume imaging; the inverted Nikon Ti2E microscopes allow diverse options for automation routines – using JOBS, and have been equipped with incubators chambers, CO2 gas mixers and a variety of (custom) stage inserts. the Zeiss upright systems – and the Axioscan Slide scanner service – excell for quick scans and making overiew images that can be used on confocal – using ZEN Connect. Luminescent samples can be images on our dark-box.
02. Confocal Microscopes
Confocal laser scanning microscopy is a flexible technology that suite many imaging applications. Our confocal machine park consists a diverse set of confocals micrscopes in upright (↓), inverted (↑) and vertical (↔) orientation. Different applications are available, such as Airyscan, FRAP, FLIM, as well as options to automation routines, such as ‘experiment designer’. Using ZEN Connect, widefield image data (e.g. slide-scanner images) can be opened and navigated trough to assist setup of imaging, or add multi-dimentional data-sets. Many of the confocal setups have been equipped with incubators or (custom) heated-controlled stage inserts and/or CO2 gas mixers. A dual pipette Aspiration assay has been added to the Leica Stellaris 5 inverted microscope.
03. Multiphoton Microscopes
Multiphoton microscopy relies on the simultaneous absorption of two or more photons by a fluorophore, most often using infra-red laser. Multiphoton microscopy most significan atvanteage over other technoligies is that it allows exceptionally deep light penetration with less phototoxicity. It therefore best used for ‘non-invasive’ imaging in living samples / organism. Our multiphoton system allows a very large working space, resonance scanner and allows Fluorescent Life-Time imaging applications (FLIM).
- Leica Sp8 DIVE Multiphoton + confocal (room i04.U1.018) : book in PPMS
04. Spinning Disk / Multi-point Confocal
Spinning Disk microscopes – also known as ‘Multi-point Confocal microscope’ are fast camera-based imaging systems. Spining Disk Confocals technology induces low phototoxicity and reduced photo-bleaching effects compared to point scanning microscopes and are therefore a good match for live imaging but also excel in acquisition of large data volumes (cleared tissues, expanded samples or brain sections). Most systems have been equipped with incubators chambers or temperature-controlled stages inserts, CO2 gas mixers and/or a variety of (custom) stage inserts. In addition, Spinning-disk confocal machine park allows many options to customize automation routines, as well as feed-back routines allow the triggering of external hard-ware during acquisition → please approach for custom automation project request.
05. Multi-modal multi-point Confocal
‘Multi-modal multi-point confocal system‘ are also fast camera-based imaging system – similar to our Spining Disk Confocal Microscopes – but host additional modalities, such as Widefield, Super-resolution (SRRF, SoRa) or TIRF modalities. Different modalities can be combined in both live and fixed samples of different volumes. The systems have been equipped with temperature incubators, CO2 gas mixers and/or a variety of (custom) stage inserts.
06. TIRF Microscopes
Total Internal Reflection Fluorescence (TIRF) Microscopy uses the properties of an induced evanescent wave – by exciting the sample under a ‘critical angle’ – immediately adjacent to the interface between two media having different refractive indices (RI): the contact area between the specimen (e.g. liquid, cells) and the glass coverslip or culture dish. This means that fluorophores are only excited in a very limited volume, also increase in signal-to-noise ratio from the restricted of the excitation volume. TIRF is mostly applied for ‘high resolution’ imaging application and is often combined with fluorescence photobleaching recovery (FRAP). All our TIRF setups have enclosed interlocked incubation chambers to guarantee laser safety. Most systems are equipped with temperature incubator and CO2 gas mixers.
07. Light sheet Microscopes
Lightsheet microscopy – otherwise known as selective plane illumination microscopy (SPIM) is a powerful technique for (large) volumetric imaging data with isotropic resolution. Light sheet microscopy is a fast cameras based imaging application that restricts photobleaching and phototoxicity effects. Due to these benefits, Light Sheet microscopy is often used for in-depth analyses of large, optically cleared (or expanded) samples and for long-term three-dimensional (3D) observations of live biological specimens at high spatio-temporal resolution. Lattice Lightsheet Microscopy is aimed for fast sub-cellular volumetric imaging – for prolonged periods.
- Zeiss Lightsheet 7 Microscope (room i06.U1.405)
- Zeiss Lattice Lightsheet 7 Microscope (room i06.U1.405) – coming soon
08. Force probing equipment
- JPK CellHesion Atomic Force Microscope (room i24.U1.301)
- Impetux Sensocell Optical Tweezer (room i04.U1.019) – coming soon
09. Stereo Microscopes (No Charge)
As part of the Imaging and Optics Facility infrastructure, a number of stereo microscope are available for sample-inspection and -mounting near to our imaging setups. Two stereo microscopes are equipped with an monochrome camera.
IMAGE ANALYSIS
The Imaging and Optics Facility provides an sate-of the art image analysis infrastructure that includes a large number of high-end analysis workstations as well as a broad spectrum of different image analysis software applications.
In addition, we provide project based solutions for custom-tailored image analysis tasks: we help you to automate your image analysis workflow, or establish new image analysis routines using the image analysis programs available. You can find them on our GitLab page or request our support via PPMS.
10. Image Analysis Workstations
Our workstations machine park consists of 6 PCs and 5 Virtual Machines (VDI), where VDI machines are based on a ‘golden image’ – that is shared over multiple workstations. Analysis workstations are specified for specific image analysis applications: ‘classical image analysis‘ & ‘machine learning-based‘ analysis. All our workstation can be accessed from remote, and a large portion can also be used from dedicated ‘connection terminals’ in room i01.O3.52a/b.
11. Image Analysis Software
Workstations (both PCs and VDIs) have preinstalled software, where some ‘propriety software’ will need to be booked separately – as resources are limited by the number of licenses available. You can find an overview list here.
AUTOMATED RENTAL SYSTEM
The Imaging and Optics facility released an “Automated Rental System” for Experimental Accessories, Optical Tools, (high-end) Objectives. The Automated Rental System helps to coordinate access-rights and the utilization of the available rental items and allows the Imaging and Optics facility to make the rentals available during the weekends → A brief trainings is required to get booking rights.
